Common Ocular Peer Review Dialogues (Part 1)
A common ocular peer-review dialogue concerns the presence of controls and sham animals.
Often, proof-of-concept studies lack appropriate control tissues, and this makes the interpretation at histopathology very challenging. Short-term studies often lack sham treated ocular tissues as well which makes it difficult for the pathologist to differentiate the potential experimental changes from the real changes which could be induced by the test article. Control eye tissues should be included in the actual study and it is not recommended to use control tissues from a colleague or from a previous study just because of convenience.
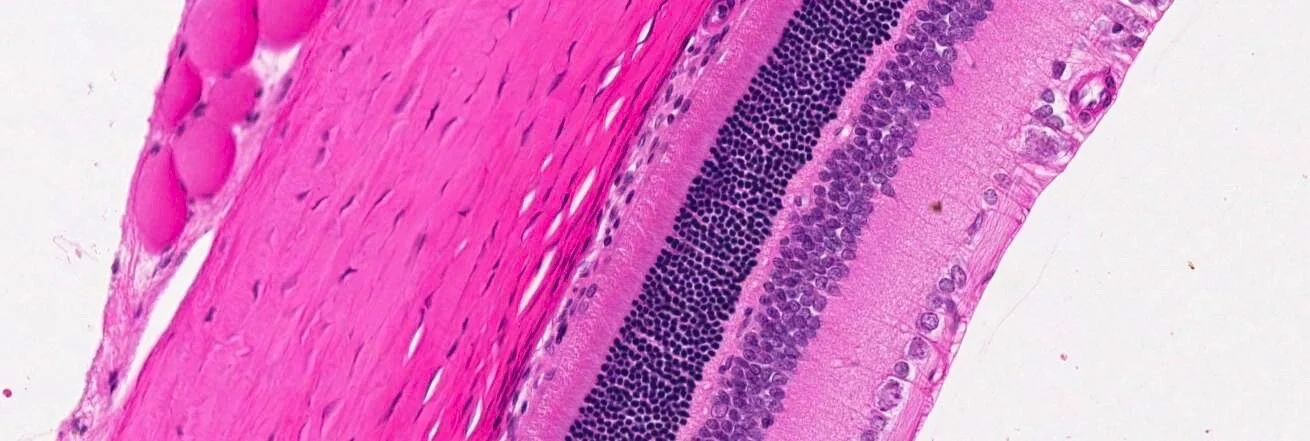
Another common ocular peer-review dialogue concerns the orientation of the ocular globe. We need to be able to localise the change related to the test article or the change related to the experimental related procedure with precision. When the orientation is lacking during the histopathological evaluations, the study protocol contains or should contain enough detail to reconstruct the orientation. Unfortunately, when major orientations data or notes are skipped during the process especially at necropsy it could be well that the orientation is lost irreversible. In the worst case we still have the anatomy or the position of the ciliary artery which can help us out.
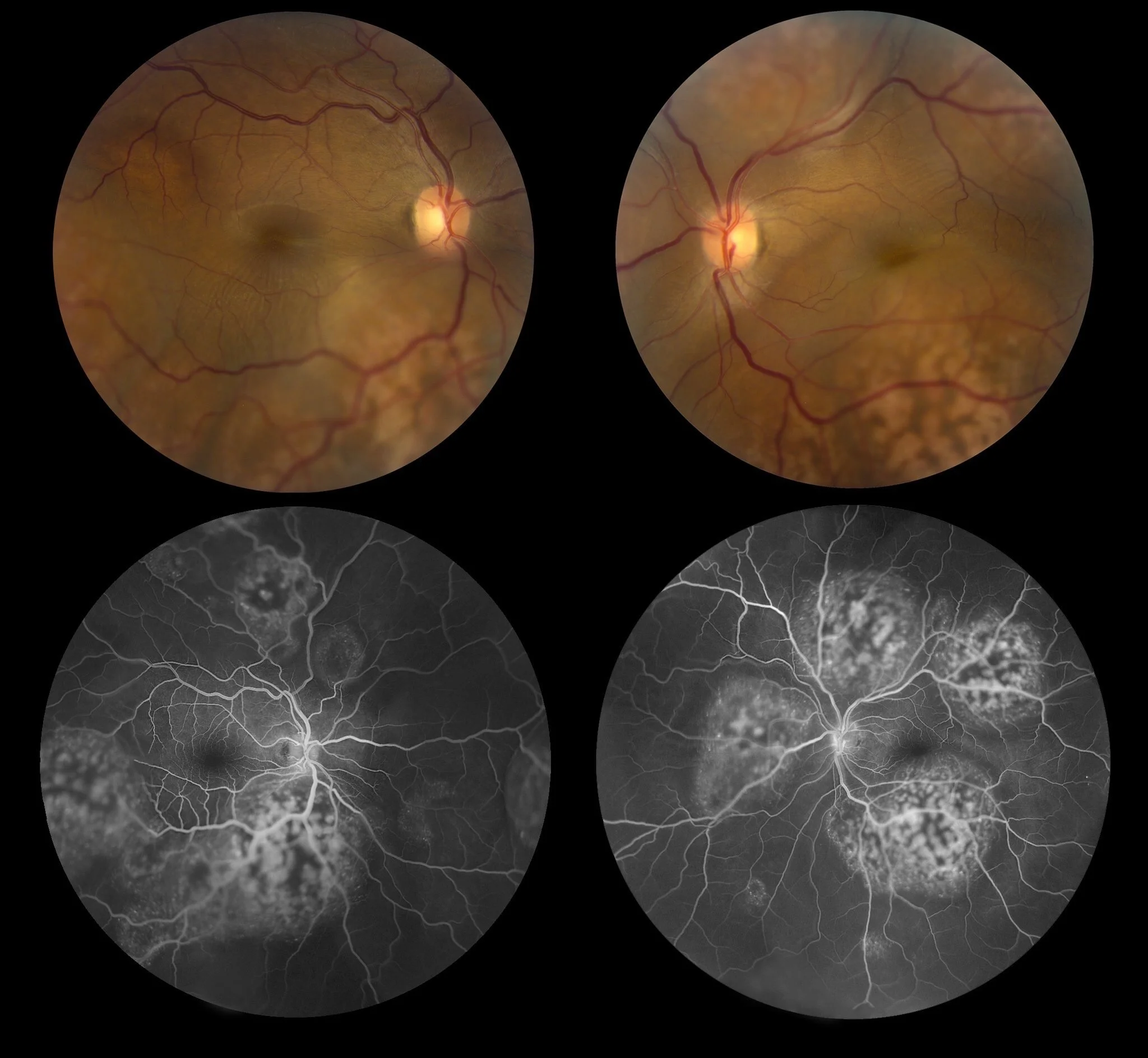
One of my favorite common dialogue is the correlation between the in vivo and postmortem because translational ocular pathology requests close collaboration between clinician’s, study directors and pathologists. One of the types of in-vivo investigations which I found very useful is the fundus examination, but also advanced technology such as the OCT. This technic provides a great advantage because the cross-sectional retinal details are collected with high precision. There are many advantages of the in-vivo exams compared to the pathology.
There is one dialogue we cannot skip in each peer review because it is always about terminologies. Terminologies may vary depending on the type of study. We often use the INHAND terminologies as they provide consistency through our communities and so, we have strong basis to compare our findings with Historical Control Data bases. In ocular pathology we have for sure common changes, but we still may use specific terminologies depending on the unique ocular pathophysiology and type of study and animal species.
During my major peer reviews but also my pathology education I have been trained to differentiate real changes versus background changes versus procedure related changes. This is a big one and the question here is how you will achieve the best interpretation of a change; As there is no miracle, there are several steps to consider in order to achieve your goal. Know your background data - If you want to differentiate the test article related change from a spontaneous background change you need to know the potential confounding factors including potential artifacts and / or experimental related procedural changes – please keep also in mind that both interpretations are sometimes possible as the test article may have exacerbated the initial background change.
A crucial dialogue and in my opinion one of the most difficult one is the discussion about adversities. Again, there is no one straightforward answer to this – so, we will go by steps - Again, there is no one straightforward answer to this – so, we will go by steps - Again, there is no one straightforward answer to this – so, we will go by steps - A few diagnoses are red flags such as lens opacification's, neurites, advanced inflammatory changes, and neoplasia’s. Always try to keep in mind that IND submissions will serve for a clinical trial - in the best-case FDA will request additional eye exams during trials to guarantee the vision safety of the patients.
The dialogue about the pathology report is one of the most important ones. We need to continue thorough communication at all stages of the ocular study and these needs to be conducted with all stakeholders putting them at the same level of responsibility. Make sure that the study design is comprehensively explained in the pathology report. Be careful when you include pictures; there is absolutely No need to repeat in a report for me that all structures were normal.