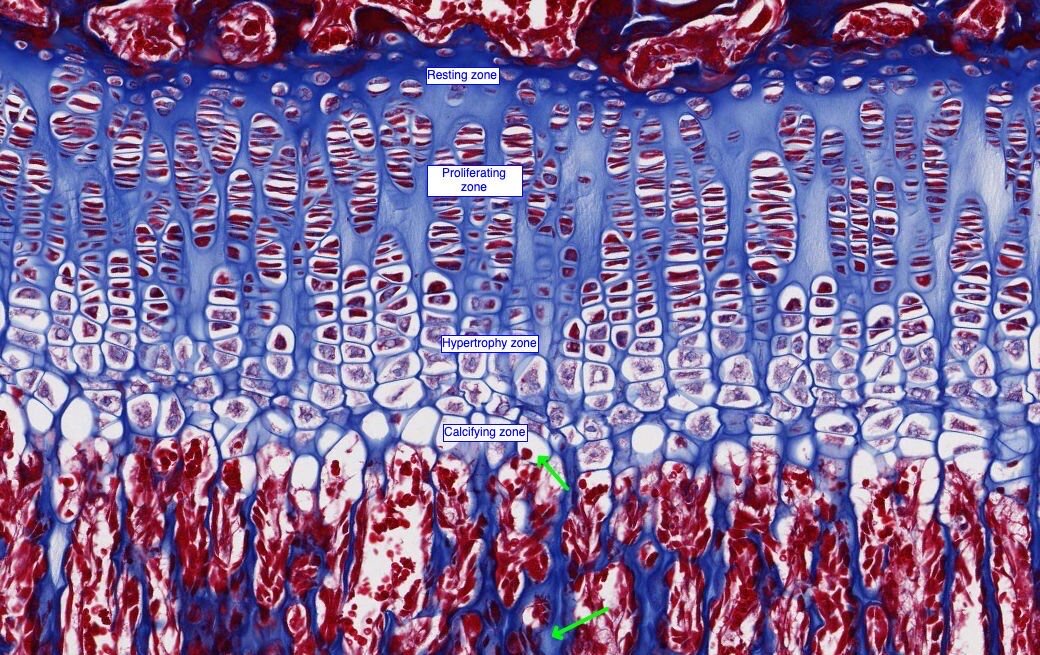
Digital Pathology Peer-review during COVID-19 times ? (Part 1)
In order to answer this question, it’s important to remember the goals and outcomes of a peer-review. The Pathology Peer-review is the process of obtaining a second opinion to confirm and potentially improve diagnoses and interpretations from the study pathologist. When performing preclinical studies within a regulated GLP environment, the peer-review process needs to comply with the actual OECD or FDA regulations. Although many of us, are dealing with non-GLP studies often similar practices will be applied in order to guarantee the accuracy and reproducibility of the (raw) data).
Two major peer review Categories can be Distinguished.
The most Common one is called the contemporaneous or prospective peer review process.
the peer review pathologist reviews the data of the study pathologist and a discussion is started to confirm or reach a consensus.
the pathology report has not been signed yet.
this type of peer review can be conducted on site, or also remotely
In my opinion, this is the most common and most straight forward peer review process.
the second type of peer review is called the retrospective peer review process.
The pathology report has been signed and is considered the raw data of the study.
this type of peer review is common during due Diligence processes and can be conducted on site or remotely
re-opening the histopathological evaluations means it has to be documented with a protocol amendement in which the methodology needs to be described in detail incl. the reason, and which types of organs/tissues will be reviewed.
Both types of peer reviews can be conducted on site, or remotely with or without using digital pathology tools. In general, the glass slide remains the reference also called the specimen from a Quality assurance point of view. If Whole slide image files are used, it should be specified in the study protocol. In most cases, the Digital image files are not needed to be archived but of course a few exceptions exist or more will come !
PRIMARY HISTOPATHOLOGICAL EVaLUATIONS
when the digital peer review concerns primary histopathological readings, and no amendement had to be implemented, there should be no concern when using the WSI as long as any potential Discrepancy that may arise during the peer review process is resolved by using the glass slide.
when the digital peer review is requested during a due diligence process while using WSI images, the GLP requirements may change because as mentioned above, the organs/tissues should be documented as well the WSI files. the retrospective peer review is prone to minor/major changes which may impact the outcome of the study and the previously established NOAEL’s. in the actual regulatory context, WSI files should be archived in order to respect the Bascis requirements of GLP: the ability to reconstruction the study from its end till its initiation.
quantitative measurements, morphometry, algorithms and artificial intelligence
finally, when the goal of the study is not primary histopathological evaluation anymore, but the focus includes quantitative measurements, morphometry with additional Artificial intelligence tools such as Algorithms, it’s becoming evident that all WSI files should be archived as the initial data were Extracted from the glass slides. from a quality assurance perspective, these initial tissue spots need to be identified and archived according the state of art GLP regulations.